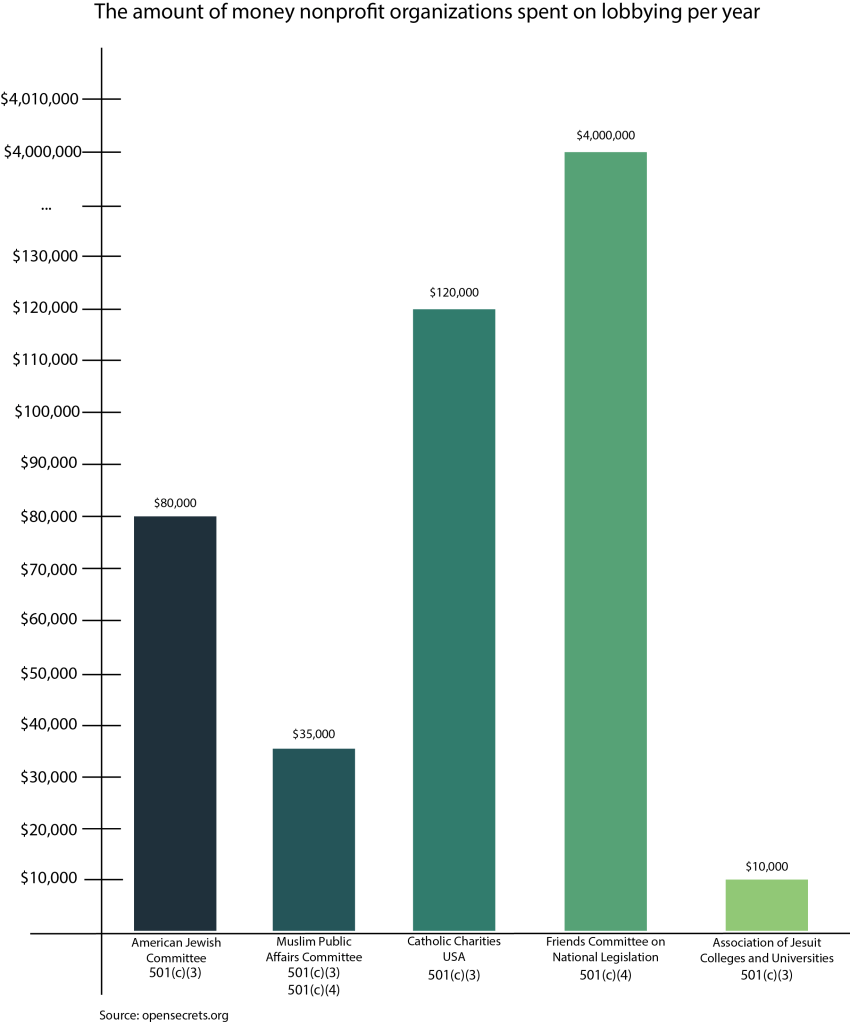
Senators expressed frustration with the “unacceptable,” in the words of Sen. Patty Murray (D–Wash.), COVID-19 testing capability of the American healthcare system to Trump administration health officials in a Senate Committee on Health, Education, Labor and Pensions hearing on coronavirus Tuesday.
Trump administration officials from the Centers for Disease Control, Department of Health and Human Services, Food and Drug Administration, and the National Institutes of Health all testified in the nearly three-hour hearing.
Murray, ranking member on the committee, represents Washington, the state hardest hit by the virus with 10 deaths from the effects of COVID-19 as of the time of the hearing.
“The administration has had months to prepare for this and it is unacceptable that people in my state and nationwide can’t even get an answer as to whether or not they are infected,” Murray said. “To put it simply, if someone at the White House or in this administration is actually in charge of responding to the coronavirus, it’d be news to anybody in my state.”
She added that with community transmission — when the virus is spread by an unknown source in the community — starting in the United States, diagnostic testing needs to be accessible to all who need it.
“People across my state, and, I’m sure, across the nation, are really scared,” said. “I’m hearing from people who are sick who want to get tested are not being told where to go. I’m hearing that even when people do get tested — and it’s very few so far — the results are taking way longer to get back to them.”
The CDC has come under criticism from Murray and others for its slow response in sending respiratory testing kits to labs around the country. While the World Health Organization focused on three genetic components of the virus’s protein crowns (from which the name coronavirus stems) to test for COVID-19, the CDC developed their own test that was quickly shown to be partially defective, according to Time Magazine.
“I thought we were better prepared for this when it happened, and it doesn’t seem to me that we were,” said North Carolina Senator Richard Burr (R), criticizing limited testing ability and efficacy in the first months of the outbreak.
“The CDC’s piece in this is to supply the public health labs with tests,” said Dr. Anne Schuchat, principal deputy director of the CDC. “We developed the test very quickly and then detected some problems after the quality control steps were measured.”
Schuchat said the CDC is now confident in its test and that public health labs would be capable of testing up to 75,000 people by the end of the week.
Schuchat explained that public health labs are only a small piece of the larger testing response to the virus. Private testing labs represent a much bigger piece of the total testing pie and will do far more testing than public labs once it is scaled up, she said. The FDA is responsible for getting those labs up and running for COVID-19 testing.
Dr. Stephen Hahn, commissioner of the FDA, said that a private company is further developing the testing platform pioneered by the CDC.
“Our expectation in talking to the company that is scaling this up, is that we should have the capacity by the end of the week to have kits available to the laboratories to perform about a million tests,” Hahn said.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said a blood test that could be performed quickly in a regular hospital clinic (rather than needing to be sent to an off-site lab) might be available soon in response to a question from Louisiana Senator Bill Cassidy (R), who is a doctor himself. This type of test would shorten wait times for results.
Alabama Sen. Doug Jones (D) told officials to push information to the public as quickly as possible, citing anxiety about the virus among the general population.
“We’re about to head into the allergy season, as well, and I just can tell you that people are so scared out there right now that the first time they sneeze with an allergy, they’re going to think that they’ve got [COVID-19],” Jones said. “We need to make sure that we try to educate folks so that those tests that we have, those limited ones, are for the right reasons.”
While testing for the virus should be more widely available by the end of the week, treatments and vaccines have a much longer timeline, according to Fauci. Both have yet to be fully developed and will require extensive safety testing before being deployed for use in the general public, according to the officials. The vaccine is at least a year away, Fauci said.
Fauci also said Gilead Sciences, a California-based pharmaceutical company, has a potential treatment for the disease called Remdesivir, which is being tested in both China and the United States.
“We should know within a period of several months whether or not this particular drug works,” Fauci said. “If it does, the implementation of that would be almost immediate.”